Stay Informed
Follow us on social media accounts to stay up to date with REHVA actualities
|
|
|
|
|
Ilia Kravchenko | Risto Kosonen | Simo Kilpeläinen | Sami Lestinen | Pertti Pasanen |
Doctoral candidateAalto Universityilia.kravchenko@aalto.fi | ProfessorAalto Universityristo.kosonen@aalto.fi | D.Sc., laboratory managerAalto University simo.kilpelainen@aalto.fi | D.Sc.Aalto University and A- InsinööritSami.Lestinen@ains.fi | ProfessorUniversity of Kuopiopertti.pasanen@uef.fi |
The demand for improved ventilation systems is essential to ensure clean and healthy indoor air. However, these systems can encounter such challenges as microbial pollution during their operation. To guarantee adequate Indoor Air Quality (IAQ) within the European Union, guidelines like EN 15251 and EN 16798-1 have been established, offering criteria for indoor environmental quality and building energy efficiency, which have been incorporated into the building codes of member countries (Ahola et al., 2019). Although, building's ventilation system still may encounter operational challenges over the building's lifespan.
One of the challenges is microbial growth resulting from dust accumulation, water condensation, and related factors, which may occur while is not operating or when airflow is presented (Pasanen et al., 1993). Polluted ventilation systems may emit microbial colonies into the indoor air, potentially leading to mold allergies and fungal diseases (Al-Doory and Domson, 1984; Sakamoto et al., 1996). Hence, it is crucial to comprehend the risk of bacterial and fungal contamination within various segments of the ventilation system.
In Finland, public buildings constitute approximately 30% of the country's total employment infrastructure. These buildings may be vulnerable to contamination within their ventilation ductwork as buildings remain unoccupied for more than 50% of the time, prompting the consideration of strategies to mitigate contamination risks. One approach involves halting ventilation during unoccupied periods potentially fostering fungal and bacterial growth. Another option is to maintain a minimum airflow rate continuously, although this may lead to unnecessary energy consumption during unoccupied periods.
The investigation aimed to explore how intermittent and continuous ventilation strategies during unoccupied periods could influence the risk of microbial growth in the ductwork. The study utilized a mock-up consisting of two separate ductwork systems, simulating intermittent and continuous ventilation strategies. Over four months, the same moisture and temperature conditions were maintained in both ductworks and microbial growth was monitored through wiping and air sampling techniques.
The ventilation ductwork setup was constructed using the commonly employed circular galvanized steel ducts, each with a diameter of 250 mm. The fan operated in both ducts for 12 hours daily, maintaining their nominal airflow rate. During the simulated unoccupied period, the fan in duct 1 was deactivated- see Fig. 1. Conversely, in duct two fans ran at a reduced supply airflow rate. Continuous monitoring of microbial growth was conducted within the experimental ductwork, characterized by air conditions of 70–90% relative humidity and air temperatures ranging from 11–14°C, including temperature measurements on the duct surfaces.
Air temperature, relative humidity, and dew point within the duct were monitored at 15-minute intervals, see Fig. 1. Specifically, air temperature and humidity were recorded at two locations within duct 0: one before the damper and the other after duct 1. In ducts 1 and 2, air temperature, relative humidity, and dew point measurements were taken at the sample hatch and downstream in duct 2, following the hatch. Surface temperatures inside both ducts 1 and 2 were also measured near the sample hatch, with surface temperature monitoring occurring in the middle of the insulation-covered sampling zone. The reference indoor air temperature, humidity, and dew point were measured on the exterior surface of duct 0. Airflow within ducts 1 and 2 was quantified using a rotary vane anemometer.

Figure 1. Schematic representation of the ductwork setup and measurement equipment.
To replicate the natural dry deposition of fungal spores, an FSSST aerosol generator was used to generate spores. This approach ensured a more uniform distribution of aerosolized contaminants throughout the duct system, providing a more representative evaluation of the risks associated with microbial growth within ventilation systems. P. brevicompactum was chosen to represent common fungi, as it can thrive in conditions where moisture is present (Kravchenko et al., 2023).
Throughout the testing period, wipe and air samples were collected at the beginning, monthly, and at the end of the experiment, see Table 1. To monitor microbial concentrations in the supply and exhaust air in case of microbial proliferation within the duct area, air samples were obtained using an Andersen impactor (Andersen, 1958), as shown in Fig. 2. The microbial analysis, including microbial counts in swab and air samples, adhered to standard operating procedures followed by the Indoor Air and Occupational Health research group at the University of Eastern Finland (“Research group of Indoor Environment and Occupational Health”).

Figure 2. Schematic representation of the ductwork sampling setup and placement.
Table 1. Sampling schedule and methods.
Sampling | Method | 11.17.2020 | 12.18.2020 | 01.18.2021 | 02.15.2021 | 03.10.2021 |
Contact, Fungal | Petri film | ● | ● | ● | ● | ● |
Air, Fungal | Anderson collector | ● |
|
|
| ● |
Air, Bacterial | Anderson collector | ● |
|
|
| ● |
In accordance with the findings, the levels of fungal spores within the ventilation duct exhibited a decline throughout the observed period, as illustrated in Fig. 3. Overall, the fungal spore counts remained quite low. The results indicate that in both test scenarios, no colonies of fungi developed on the surface of the ventilation duct. Notably, in the case of continuous ventilation, the viability of fungal spores decreased at a slightly higher rate compared to intermittent ventilation.
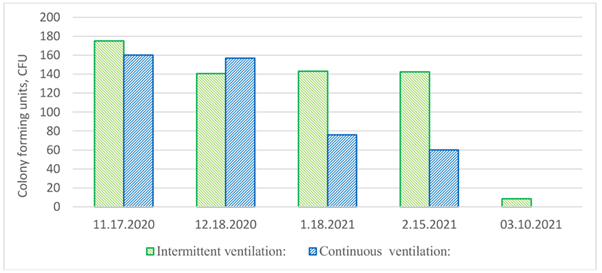
Figure 3. The changes in fungal spore counts within the ventilation duct, comparing intermittent and continuous night-time ventilation
Ductwork air samples are presented in Fig. 4 where the results of air samples obtained at the beginning (12.11.2020) and the end (10.3.2021) of the observation period, with samples collected from both ends of the ducts. Continuous ventilation consistently exhibited lower Colony-Forming Unit (CFU) counts in all instances, indicating a greater ability to withstand the transfer of bacterial colonies.
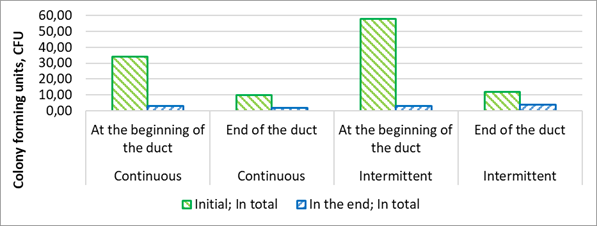
Figure 4. Air samples of bacterial colony-forming units within the ventilation ducts, comparing intermittent and continuous ventilation in the beginning of experiment and at the end.
Interestingly, the pattern of fungal Colony-Forming Units (CFU) was initially different, with colonies primarily located at the end of the duct for continuous ventilation and at the beginning of the duct for intermittent ventilation, as depicted in Fig. 5.
Overall, the air samples corroborate the findings of Petri film, indicating that the number of CFUs within the ventilation duct did not increase significantly with either intermittent or continuous ventilation operation. While the initial theory suggesting that ventilation initiation might create a pressure wave capable of distributing spores was not directly confirmed, there is a subtle difference in CFU levels presented. Based on the results from the air samples, it can be concluded that there was no observable growth within the ventilation ducts.
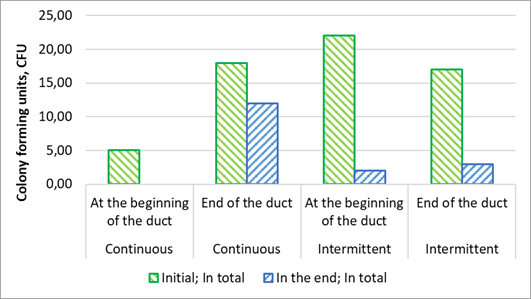
Figure 5. Air samples of ventilation duct fungi colony-forming units within the ventilation ducts, comparing intermittent and continuous ventilation in the beginning of experiment and at the end.
Both intermittent and continuous ventilation strategies have demonstrated their effectiveness in minimizing bacterial and fungal contamination. However, continuous ventilation exhibited a slightly superior performance in resisting colony growth. The experiment suggests that the likelihood of long-term bacterial and fungal colony growth on the walls of air ducts is improbable. Moreover, the consistently low colony counts in the air throughout the study period indicate that the transfer of colonies from the air ducts to other components of the ventilation system is also unlikely. These results can serve as a foundation for selecting the most suitable ventilation strategies for public buildings, ensuring the maintenance of a healthy indoor air environment.
Ahola, M., Säteri, J., Sariola, L., 2019. Revised Finnish classification of indoor climate 2018. E3S Web Conf. 111, 02017. https://doi.org/10.1051/e3sconf/201911102017.
Al-Doory, Y., Domson, J.F., 1984. Mould Allergy. Lee & Febiger.
Andersen, A.A., 1958. New sampler for the collection, sizing, and enumeration of viable airborne particles. J Bacteriol 76, 471–484. https://doi.org/10.1128/jb.76.5.471-484.1958.
Kravchenko, I., Pasanen, P., Lestinen, S., Kilpeläinen, S., Kosonen, R., 2023. Risk of Microbial Growth in Ventilation Ductwork Located in the Humid and Cold Conditions. Buildings 13, 1683. https://doi.org/10.3390/buildings13071683.
Pasanen, P., Pasanen, A.-L., Jantunen, M., 1993. Water Condensation Promotes Fungal Growth in Ventilation Ducts. Indoor Air 3, 106–112. https://doi.org/10.1111/j.1600-0668.1993.t01-2-00005.x.
Research group of Indoor Environment and Occupational Health, UEFConnect. URL https://uefconnect.uef.fi/en/group/research-group-of-indoor-environment-and-occupational-health/ (accessed 6.20.23).
Sakamoto, T., Ito, K., Miyake, M., Doi, S., Yamada, M., Torii, S., 1996. Cross-allergenicity between Aspergillus restrictus, Aspergillus fumigatus and Alternaria alternata determined by radioallergosorbent test inhibition. Allergology International 45, 45–49. https://doi.org/10.2332/allergolint.45.45.
Follow us on social media accounts to stay up to date with REHVA actualities
0