Stay Informed
Follow us on social media accounts to stay up to date with REHVA actualities
|
Peter Tomlein |
Doc. Ing. Peter Tomlein, PhD., SZ CHKT, Slovak Association for Cooling, Air Conditioning and Heat pumps, Šamorín, Slovakia |
Every advance of human society, from the first industrial revolution to the currently ongoing fourth industrial revolution, has been associated with advances in energy use and changes in technology. Whether it was the introduction of the use of steam, electricity or the automation of the production process. All these activities were connected with the use of energy, which, if we do not count hydropower, was obtained from burning wood, coal, natural gas, oil processing or nuclear fission. All the mentioned commodities represent a certain way of storing energy, which people purposefully store and use from these commodities according to the needs and demand of society. With the exception of nuclear energy, obtaining energy from the above-mentioned areas is associated with the production of resource sources (the so-called carbon footprint), which is considered the main source leading to climate change and global warming.
Humanity has set itself the goal of a carbon footprint and possibly a carbon-free society. This corresponds to the pressure to switch to ecological renewable energy sources such as solar, wind, geothermal and ambient energy. However, their fulfilment is also related to the need for energy storage, since in case of fluctuating energy production from the sun or wind, it is necessary to solve its storage in order to balance the supply and demand for energy.
In addition to biomethane, hydrogen is also an alternative and promising source of "green" energy. Its combustion produces water with the release of a large amount of energy. For the wider application of hydrogen, its cheap and ecological production is key, but above all, the possibility of safe storage. If cost-effective production and storage of hydrogen is achieved, then due to the emission-free technology, despite the lower efficiency, the use of hydrogen will be interesting. For example, in the local production of hydrogen, its storage and the production of electricity in fuel cells. Electricity would be used by heat pumps for heating and cooling.
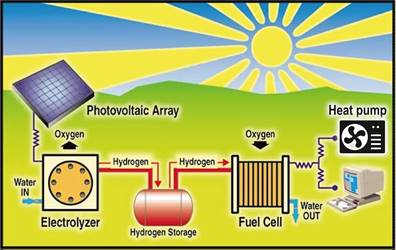
Figure 1. Integration of electrolyser, hydrogen storage, fuel cell and heat pump.
Hydrogen (H) is the lightest element on Earth. A litre of hydrogen weighs approximately 90 mg (0.09 g), so it is about eleven times lighter than air. Unlike fossil fuels (coal, natural gas...), in which energy is already accumulated and we release it from there, when using hydrogen technologies, we must first produce hydrogen by decomposing other compounds (which requires input energy in the form of work or heat) and until subsequently, either by using fuel cells or by direct combustion, release the energy from the hydrogen, so far with low efficiency.
Hydrogen, as the lightest existing molecule, also has a very low density. 1 kg of hydrogen gas, at normal room temperature and atmospheric pressure, occupies a volume of approximately 11 m³ (11,000 litres). So, with a low weight, it takes up a lot of space. It follows that for efficient hydrogen storage, we must focus on increasing its storage density, i.e. primarily on increasing the weight of the stored hydrogen (4).
From an energy point of view, the use of hydrogen is effective only if we achieve its high energy density during storage. An example to explain this phenomenon is Figure 2, which compares how much energy we can get from common fuels (gasoline, methane...) and their comparison with hydrogen. It is evident from the graph that if we take 1 m³ of these fuels (red columns in the graph), the use of hydrogen is not at all interesting, as we get the least amount of energy from one cubic meter of hydrogen. The situation changes completely if we consider weight. We get significantly more energy from one kilogram of hydrogen than from other sources (blue columns in the graph). Therefore, when developing materials and technologies for hydrogen storage, it is necessary to look primarily at how much hydrogen they are able to store.
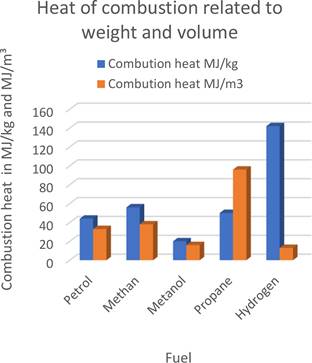
Figure 2. Energy density of common fuels and hydrogen by weight (blue bars) and by volume (red bars).
Storing hydrogen as a compressed gas is the simplest, most natural and most economical choice. This storage method is also referred to as CGH2. It involves compressing hydrogen using compressors into pressure tanks. The principle of using high pressure for hydrogen storage is shown in Figures 3a and 3b. Increasing the pressure increases the amount of stored gas and the energy density. The density of hydrogen at atmospheric pressure (1 bar) and a temperature of 20°C is approximately 0.084 kg/m³. When the pressure is increased to 100 bar and the same temperature, the density increases to 7.8 kg/m³, at a pressure of 300 bar it is already 20 kg/m³ and, for example, at a pressure of 700 bar it is almost 40 kg/m³. On the other hand, the use of higher pressure is economically more demanding in terms of energy and technology (compressors) as well as requirements for reservoirs. When using a pressure of 700 bar, the reservoirs are made of special materials, for example using expensive carbon fibres, which can make up 40-80% of the price of the reservoir.
The second practically established form of hydrogen storage as a liquid (LH2) requires very low temperatures (-253 °C) and specially insulated tanks. In addition, when liquefying hydrogen, it is necessary to pre-cool it with liquid nitrogen. Thus, the economic costs of obtaining liquid hydrogen are relatively high, on the other hand, compared to compressed hydrogen, the same amount of liquid hydrogen has a much smaller volume and higher energy density (Fig. 3c). The density of liquid hydrogen is approximately 70 kg/m³, which is almost twice as much as the density of gaseous hydrogen (40 kg/m³) at 700 bar.
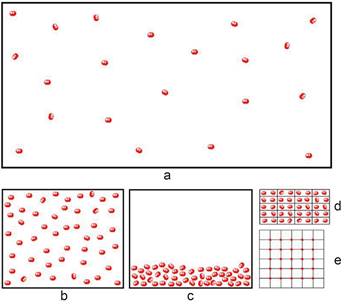
Figure 3. Illustrative representation of hydrogen molecules under different conditions: a.) hydrogen molecules in a closed vessel at normal room temperature and atmospheric pressure, b.) hydrogen molecules compressed in a pressure vessel at high pressure, c.) hydrogen molecules in a liquid state, d.) atoms hydrogen absorbed in metallic materials, e.) hydrogen molecules adsorbed in porous material (4).
It is probably not rational to assume the use of liquid hydrogen, but despite the energy requirement, liquefied hydrogen is a suitable form for transporting it to the place of use (e.g. family houses with heat pumps).
Hydrogen storage in solid structures is currently in the stage of basic research. Category of materials being investigated for hydrogen storage are hydrides (metallic or non-metallic). In these substances, hydrogen is integrated directly into the structure of solid substances and forms stronger, binding interactions with the solid substance, thus the so-called chemical sorption. In this way, it is possible to store hydrogen with a density that is not yet high. The mass storage capacity of the metal hydride is 1.43-6 %.
Hydrogen can be stored in porous carriers. For example, there is storage of natural gas on the earth's surface, where deposits are often formed by porous rocks in which natural gas is accumulated by adsorption. The key is the large specific surface area of the porous material. Modern porous materials can have a surface area of several thousand m2 per gram of carrier. The phenomenon of adsorption, therefore, leads to the "attachment" of hydrogen molecules to the surface and in the pores of the porous material. In this way, it is possible to store hydrogen with a density that is not yet high, up to 6% of the total weight.
If hydrogen were to become an integrated part of energy distribution, it will also be necessary to solve the transport and distribution of hydrogen on a large scale from the place of production to the consumer (1).
One of the existing and safe solutions is the distribution of hydrogen gas through pipelines. Hydrogen distributed in the pipeline has higher requirements for the tightness of the pipeline, the materials used. Pipeline distribution is technologically mastered. It has been used for many years. Compared to the transport of e.g. natural gas, transporting hydrogen through pipelines is more complicated and expensive due to the higher energy required to push hydrogen into the pipeline and its low volumetric energy density. This requires 3x higher gas flows. It is estimated that approximately 4.6 times more energy is needed to transport hydrogen through pipelines than to transport natural gas. In addition, significant energy losses occur during transportation, approximately 10% for every 1000 km.
In addition to pipelines, hydrogen can also be transported in pressure cylinders with a pressure of up to 350 bar or 700 bar. However, with the expected high consumption of hydrogen in the case of the introduction of hydrogen technologies, such distribution would be slow and uneconomical.
An alternative is to transport liquid hydrogen, which is difficult to store due to evaporation. Liquefaction is a time- and energy-consuming process and takes place at a temperature of -253 °C. The advantage of liquid hydrogen is its high energy density, which is three times higher than that of gasoline. Only nuclear fuel has a higher energy density.
An alternative is the transport of hydrogen bound in compounds, e.g. molecular hydrides, mentioned in the previous chapter.
Hydrogen houses can be divided into houses with their own hydrogen production, houses buying liquid, gaseous or solid hydrogen and houses connected to a gasification network with a mixture of natural gas and hydrogen, later also potentially pure hydrogen (1). Combustion of hydrogen is energetically inefficient and therefore the development deals with the economics and energy efficiency of the integration of the production, storage and use of hydrogen in fuel cells for the production of electricity. Electricity can be used not only for heating, but also for cooling the house.

The efficiency of hydrogen production is low, less than 60%. It will increase around 90 % only if we use the heat released during the electrolysis of water. Moreover, the building cannot be cooled by burning hydrogen.
60 kWhe are consumed to produce 1 kg of hydrogen with a combustion heat of 39 kWth. If 60 kWhe is used directly in the heat pump, 6 times more up to 240 kWhth heat is produced. The burning of hydrogen has a very low efficiency, and the heat produced is at least 5-6 times less than the heat produced by the available electricity directly by the heat pump.

It is likely that hydrogen production will be decentralized and integrated into hydrogen technologies. In that case, the physical transport of hydrogen to such facilities will not be necessary. They will work autonomously with the potential use of heat pumps. The economics of such hydrogen projects currently do not exist.
In the case of hydrogen production by electrolysis, which is then stored and used to produce electricity in the fuel cell, which is used to drive the heat pump, the resulting efficiency of the integrated solution does not reach the energy efficiency of heat production by the heat pump with electricity from the electrification system. However, the efficiency of the electrolysis, fuel cell and heat pump combination can be increased if the heat released in the electrolysis and in the fuel cell is used. Due to the lower efficiency and significantly worse economy, such an integrated technical solution is currently and in the near future problematic.
It is limited by the availability of green electricity, which limits the operation of the electrolyser with a power of 75-100% only for a period of 20% of the year. Conversely, the unavailability of green electricity above the technological limit of the electrolyser’s performance (20%) puts the electrolyser out of operation for more than 60% of the year. The starting point should be the production of the so-called of low-carbon hydrogen with partial use of electricity from the distribution network with the assumed condition of ensuring at least 70% less greenhouse gas emissions than fossil natural gas during the entire life cycle.
We look at the application of hydrogen from the point of view of heat pumps from the point of view of the structure of the energy system and the need for heating and cooling buildings. Hydrogen is needed mainly for industry, transport and the sustainability of the gasification network. For now, hydrogen is in short supply and it is likely that it will have to be imported, thus only partially fulfilling the requirement of energy independence. The production of hydrogen for the gasification network, first by mixing and assuming the transport of pure hydrogen, would probably require a demanding renewal of the network in order to prevent large losses and reduce the multiple energy requirements of transport.
If hydrogen could be economically produced, stored in sufficient quantity and transported with adequate energy density, then integration with fuel cells and with a heat pump for both heating and cooling would come into consideration. Even this solution is not more energy efficient than the direct use of electricity by a heat pump and is still many times more expensive.
It follows from the above that the production of hydrogen for the purpose of heat production by combustion is energetically and economically inefficient and therefore unsuitable. The efficiency of the combination of electrolysis, hydrogen storage, fuel cells and heat pump can be increased if the heat released in electrolysis and in the fuel cell is used. Due to the lower efficiency and significantly worse economy, such integrated technical solutions are unlikely at present and in the near future, because the economy in hydrogen projects does not exist at the moment.
1. Illith, R.: Pilot project of heating the village with a mixture of natural gas and hydrogen. H2 heating. SPP Bratislava. Apoks, Praha, May 2023.
2. Norman Gerhardt et al: Hydrogen in the energy system of the future. Focus on heat in the building. Frauenhofer IEE May, 2020. https://s.fhg.de/GV4.
3. Sodomka V.: Hydrogen as alternative fuel. In: TZB Haustechnik, Bratislava 02/2023 p. 42-45.
4. Zeleňák, V., Ivan Saldan: Factors Affecting Hydrogen Adsorption in Metal–Organic Frameworks: A Short Review, Nanomaterials, special Issue, 2021.
5. Zeleňák, V.: Storage and transport of hydrogen as a key factor for the development of hydrogen technologies. UPJŠ Košice, 2023.
Follow us on social media accounts to stay up to date with REHVA actualities
0